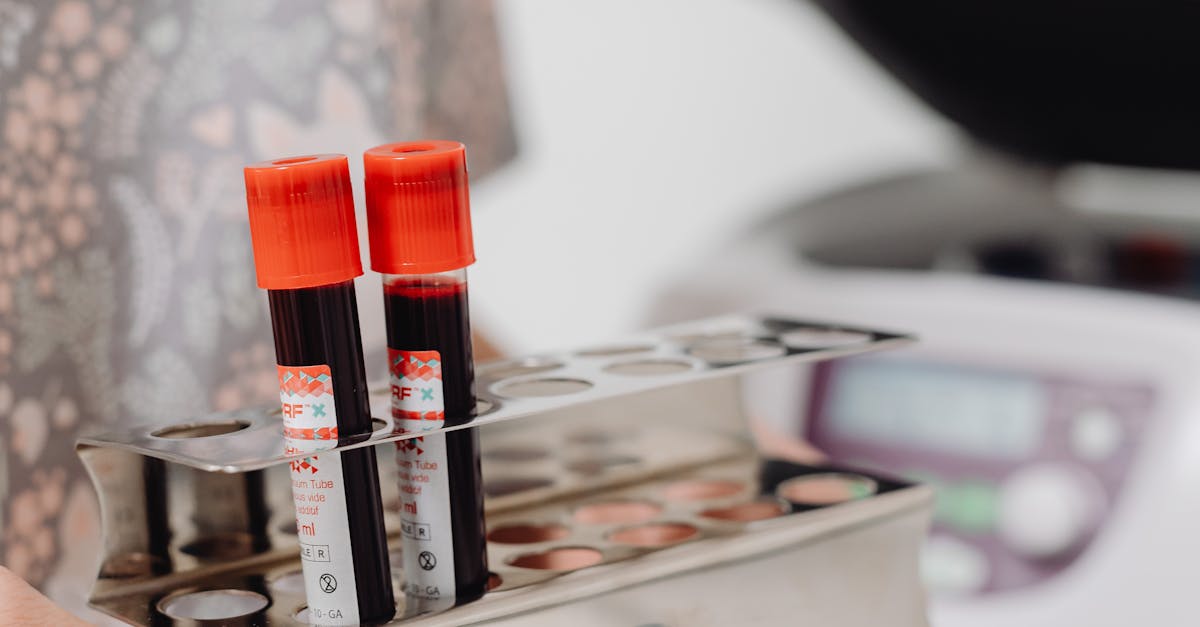
What does rt mean in chemistry?
The prefix “r” refers to the gas, or radical, hydrogen. When speaking of chemical reactions, the “r” designates the gas – or radical – produced by the reaction in question. The symbol “t” stands for “turn” or “transformation”, and is used to designate a reaction that produces a new chemical or a change in the structure of the reactants.
What did the word rt mean in chemistry?
rt means reaction temperature. It was first used in the work of French chemist Henri Victor Bois, who also developed the concept of equivalent weights. The term was first used to describe the temperature at which a reaction occurred in an effort to standardize the temperature at which reactions occurred in different labs.
What does the number rt mean in chemistry?
The letter rt is a prefix that is used to represent the gas constant. The gas constant describes how much energy is required to increase the temperature of a gas by one degree Celsius. It is defined as the average energy required to increase the volume of a gas by one mole; one mole is equal to the amount of atoms in a sample of 1.5 × 10−23 g.
What does the symbol rt mean in chemistry?
The symbol rt stands for reaction temperature. It is used when the change in temperature is absolutely constant. In other words, the temperature does not change during the reaction. You will see the symbol on a reaction that takes place at a specific temperature, such as 100°C.
What does the acronym rt mean in chemistry?
The word “rt” stands for reaction time, so the rt value of a reaction is the time, in seconds, that it takes for the reaction to occur. This does not refer to the time of the day or the year when the reaction occurs, but rather the time it takes for the reaction to complete itself.






